[ad_1]
November 17, 2023
4 min browse
The look for for space-temperature superconductors has endured scandalous setbacks, but physicists are optimistic about the field’s upcoming
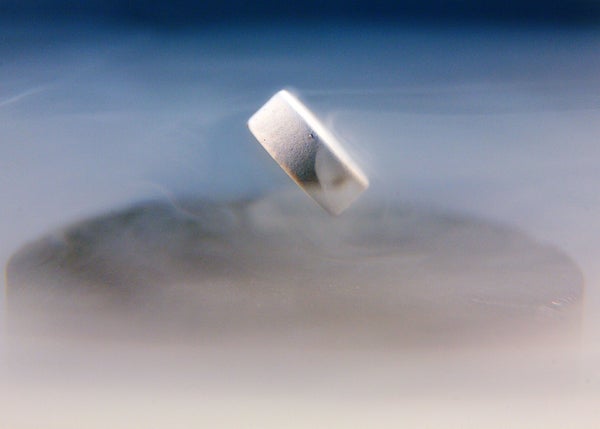
A magnet levitating around a nitrogen-cooled superconductor.
A Mother nature retraction past week has place to rest the most recent claim of place-temperature superconductivity — in which scientists claimed they experienced built a content that could carry out electrical energy with out creating squander warmth and with no refrigeration.
The retraction follows the downfall of an even more brazen assert about a supposed superconductor named LK-99, which went viral on social media previously this 12 months.
Regardless of these superior-profile setbacks, superconductivity researchers say the subject is savoring fairly of a renaissance (see ‘Timeline: Superconductivity milestones’). “It’s not a dying field — on the contrary,” states Lilia Boeri, a physicist who specializes in computational predictions at the Sapienza University of Rome. The progress is fuelled in part by the new abilities of laptop simulations to predict the existence and homes of undiscovered resources.
Significantly of the excitement is focused on ‘super-hydrides’— hydrogen-wealthy supplies that have proven superconductivity at at any time-larger temperatures, as long as they are stored at large force. The issue of the retracted Nature paper was purported to be this sort of a product, produced of hydrogen, lutetium and nitrogen. But function in the previous number of years has unearthed numerous people of resources that could have innovative properties. “It genuinely does glance like we’re on the furry edge of becoming capable to obtain a whole lot of new superconductors,” claims Paul Canfield, a physicist at Iowa Condition College in Ames and Ames National Laboratory.
Browsing electrons
Superconductivity occurs when electrons in a strong blend to kind ‘Cooper pairs.’ This permits lots of more electrons than typical to transfer in sync within the content, which in change enables the electrons to carry currents with out making waste heat.
In ‘conventional’ superconductors, electrons variety Cooper pairs when nudged alongside one another by vibrations in the product — mechanical waves that the Cooper pairs journey like surfers on a wave. Right up until the mid-2000s, scientists commonly considered that this system would work only at very reduced temperatures, up to all around 40 kelvin. Superconductors produced of a solitary component all call for temperatures lessen than 10 kelvin to show this home. Magnesium diboride, a conventional superconductor uncovered in 2001 by a workforce led by Jun Akimitsu at Okayama University in Japan, lifted the file for the optimum temperature to 39 kelvin.
The foundation for super-hydrides was laid out in 2004, when the late theoretical physicist Neil Ashcroft predicted that specific aspects would form compounds with hydrogen that could superconduct at substantially increased temperatures than could any other materials, if place below ample stress to power the hydrogen atoms closer together.
In accordance to Ashcroft’s theory, the proximity of the hydrogen atoms would improve the frequency of mechanical vibrations, which would enable the material to get warmer when retaining its superconductivity. But there was a catch: to even exist, some of these materials would require pressures similar to all those in Earth’s main.
Innovations in carrying out significant-tension experiments on tiny samples inside a diamond anvil — and measuring their results — led to a breakthrough in 2015, when physicist Mikhail Eremets at the Max Planck Institute for Chemistry in Mainz, Germany, and his collaborators very first demonstrated superconductivity in a tremendous-hydride, hydrogen sulfide. Given that then, scientists have predicted the existence of many other superconducting resources in this family — some of which have been uncovered, which includes calcium-based cage-like constructions known as clathrates.
At existing, the ‘hottest’ superconductor of any kind is thought of to be lanthanum decahydride, a member of the tremendous-hydride course that is tested to be a substantial-strain, traditional superconductor at temperatures of up to at least 250 kelvin.
Superior simulations
Eremets and others say that the interplay of theory, simulation, elements synthesis and experiment has been essential to progress. Starting in the early 2000s, it grew to become feasible for simulations to predict no matter if a product with a sure crystal framework and chemical composition could be a superconductor, and at what temperatures it could exhibit this residence. But the next major change was the introduction of algorithms afterwards that ten years that could predict not just the houses of a materials, but what resources can variety from a presented mix of aspects. “Until then, a very important bit was lacking: understanding whether a compound can sort in the initial spot,” says Boeri.
The discovery in 2015 that hydrogen sulfide is a superconductor was steady with computer system simulations done the year just before. With out fast developments in framework prediction, the discovery of hydrogen-prosperous superconductors “probably would have not happened for one more century,” says Artem Oganov, a supplies scientist at the Skolkovo Institute of Science and Technologies in Moscow, who has pioneered construction-prediction algorithms. His ‘evolutionary’ algorithms, in certain, locate the configuration of atoms with the lowest power — and thus finest prospect to type and continue to be steady — at a offered tension.
Simulations are specially important for predicting the behaviour of supplies at large pressures, less than which atoms are pushed so near to 1 another that they commence to interact not only through their outer electrons, but also with more internal ones, throwing chemistry-textbook dogma out of the window. An illustration of this is lithium hexahydride, which can exist only at significant pressures. “Anybody in standard-chemistry course would inform you that anything like LiH6 cannot be stable,” says Eva Zurek, a computational chemist at the University at Buffalo in New York.
This write-up is reproduced with permission and was very first published on November 16, 2023.
[ad_2]
Source url